Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Deficiency In Regulation of DNA Damage Repair in Neurons May Open Long Path At Middle Age To Dementias At Old Age
*Corresponding author:Yahuan Lou, Department of Diagnostic Sciences School of Dentistry, University of Texas Health Science Center at Houston, Houston, TX 77054, USA.
Received: January 11, 2024; Published: January 22, 2024
DOI: 10.34297/AJBSR.2024.21.002821
Abstract
Dementias in seniors including sporadic late-onset Alzheimer’s disease have become a significant socioeconomic burden worldwide. It could be resolved by their early diagnosis and intervention before irreversible neuron damages. Thus, to elucidate a common cause for those dementias is critical. Recent studies have questioned whether accelerated aging process in neurons may be responsible due to deficiency in neuronal rejuvenation of aging neurons such as DNA double strand breaks (DSBs). Aging causes oxidative DAN damages. Deficiency in DSB repair leads to accumulation of DSBs in neurons especially after middle age. Due to nonproliferative nature, aged neurons must be rejuvenated to keep their functionality for nearly whole lifespan. DNA integrity is critical to ensure the rejuvenation. We have recently shown that activation of interlukin33-ST2-NFκB axis in neurons initiates DSB repair. Thus, deficiency of this axis in neurons impairs DSB repair. Furthermore, unrepaired genomic damages disrupt other neuronal rejuvenation mechanisms, leading to chronic development of neurodegeneration in aged mice. It would be interesting to investigate if it is the case in humans.
Failure In Rejuvenation of Aging Neurons May Be a Common Cause for Senile Dementias
Senile dementias, mainly late-onset sporadic Alzheimer’s disease, are an increasing socioeconomic burden worldwide. All those dementias are characterized by chronic neurodegeneration without significant symptoms. Thus, upon the diagnosis, they are often irreversible. Despite of decades’ intensive research, their cause is still unclear. Recent investigations begin to ask whether abnormal neuronal aging is a possible cause for chronic neurodegeneration. Recent studies have suggested that new neurons in certain brain areas could be generated from stem cells. However, majority neurons are considered non-proliferative, and thus, cannot be replaced through cell division. Therefore, healing or rejuvenation of stressed neurons during aging is vital for maintenance of brains’ functionality up to old age. Mounting evidence supports that senile dementias may result from either accelerated aging process or impairment in rejuvenation of aged neurons [1-5]. Several mechanisms have been identified in neurons, which may rejuvenate stressed neurons.
Those include repair of aging-associated DNA damage, autophagic digestion of damaged or old neuronal proteins, and glymphatic drainage of abnormal neuron-associated proteins/wastes such as degraded amyloid peptides [6-10]. In fact, linkages between defects in those mechanisms and senile dementias, especially late-onset sporadic Alzheimer’s have been either partially demonstrated in several animal models, or statistically established by clinical observations. Both type of Alzheimer’s disease, i.e. familial early-onset or sporadic late-onset, show the same characteristics (i.e. tauopathy and amyloid plaques). While structural alteration of those proteins due to mutations in genes such as APP and MAPT has been blamed for early-onset type, it is still a puzzle for late-onset type, in which APP or MAPT, or genes related to their process are normal.
Single rejuvenation mechanism such as has been linked to Alzheimer’s or related dementias, but so far animal models with deficiency in one mechanism (e.g. autophagy or glymphatic) do not develop any Alzheimer’s like symptoms in normal mice. On the other hand, the deficiency indeed accelerates Alzheimer’s symptom in mutant human APP or MAPT transgenes. Those results are not only encouraging, but also raise a possibility that deficiencies in multiple rejuvenation mechanisms may be required to cause senile dementia or Alzheimer’s disease even without mutations. What would cause deficiency in multiple rejuvenation mechanisms? One potential scenario is that an up-stream regulatory factor may govern overall neuronal rejuvenations, and thus, the regulation deficiency may impair all or multiple neuronal rejuvenation mechanisms.
Does Unrepaired DNA Double-Strand Breaks (DSB) In Aged Neurons Impair Their Rejuvenation?
Like any cells, accumulation of oxidative damages in various biomolecules is an essential part of neuronal aging [11,12]. Increasing DNA damage with age has been reported in the brains of the mouse, rat, gerbil, rabbit, dog, and human [13-16]. We have shown a sudden oxidative damages surge in the cortical/hippocampal neurons at middle age in mice (40 weeks ≈ human 46 years). Importantly, the surge of oxidative damage was absent in any glial cells, or in other organs [17,18]. Interestingly, surge of DNA damages or associated genes in brains has also been described in humans [13,15,16,19,20]. Oxidative damages of DNA i.e. AP (apurinic/apyrimidinic) lesions cause DNA double-strand breaks (DSB). Those DSBs must be repaired immediately. Otherwise, accumulated DSBs would trigger genomic instability, which incapacitates stressed neurons to function normal or induces immature death.
Several studies have reported accumulation of DSBs in Alzheimer’s brains [1,2,21-25]. Although it needs to be demonstrated when DSBs start to buildup in humans, it is less doubtful that accumulated DSBs would initiate a chronic path to neuronal malfunction, neurodegeneration and eventually dementia at old age. If it is proven, middle age, when long before onset of dementia symptom or significant pathological changes, may be a critical time window for early diagnosis or intervention of senile dementia long before irreversible neuron loss. In fact, DSBs-related genomic instability in neurons has been postulated as a potential cause for late-onset Alzheimer’s disease. Two different possible pathways may lead to accumulation of DSBs in neurons:
abnormally accelerated generation of DSB, or
failure in DSB repair. However, our study demonstrated that failure in DSB repair may be a major reason for DSB accumulation in neurons after middle age, at least in mice [17]. DSB repair mechanisms have been well elucidated, and ubiquitously exist in any types of cells. Thus, it is likely that the failure in DBS repair is not due to repair mechanism itself, but rather by neuron-special upstream regulative element. Which molecules or pathways regulate or initiate DSB repair in oxidatively stressed neurons?
Interleukin33-ST2-Nfkb Axis Is Required for Initiating DSB Repair in Neurons
Interleukin33 (IL33) is a member of the interleukin1 cytokine family [26]. After cleavage of original IL33 in nuclei (nIL33), a mature cytokine domain (cIL33) is released (Figure 1). Through binding to its receptor ST2 on target cells, IL33 triggers activation of NFκB transcription pathway [27]. Soon after its discovery, IL33 was considered a cytokine or alarmin in immune regulation and/or immune response. However, constitutive expression of IL33 in a wide range of tissues or cells such as the brain implies its roles beyond immune system [28-31].
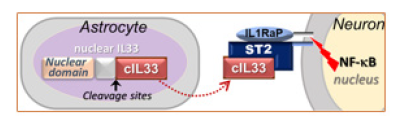
Figure 1: Lou Deficiency in Regulation of DNA Damage Repair in Neurons May Open Long Path at Middle Age to Dementias at Old Age.
It was found to participate in the injury healing in central nervous system, other diseases such as cardiovascular diseases, and tissue modification [32-35]. IL33 has been genetically linked to human Alzheimer’s disease and shows a beneficial effect in a mouse Alzheimer’s model [36,37]. Our previous study has demonstrated the role of IL33 in tissue homeostasis in ovaries [38]. Importantly, we also found an unusually high level of IL33 proteins in astrocytes. Up to surprisingly 75% of astrocytes in some brain areas contain nIL33 at old age, and cIL33 is constantly release in brains [17]. On the other hand, we detected expression of ST2, as well activation of NFκB pathway in neurons in NFκB/luciferase reporter transgenic mice (unpublished data). Thus, an IL33-ST2-NFκB axis exits between astrocyte and neurons in brains (Figure 1). Several studies on Alzheimer’s disease supported an IL33-ST2-NFκB axis in human brains. IL33-NFκB axis has been detected in brains of human Alzheimer’s disease [39].
We have tested IL33 function in brain. Using IL33 deficient (Il33-/-) mice, we found that astrocyte IL33 is critical for controlling sudden oxidative damages at middle age in neurons, and its deficiency in mice caused abnormal tau deposition and late-onset neurodegeneration in the cerebral cortex and hippocampus, followed by cognition and memory impairment at old age [17,18]. Our recent preliminary data showed that IL33 deficiency leads to generation of amyloid plaques in human wild type APP transgenic mice. The above results raise a possibility that astrocyte IL33 may be the upstream regulatory factor that governs overall neuronal rejuvenation. Our studies seem having partially answered the question. First, IL33-deficiecy in mice causes a rapid accumulation of DSBs in neurons after the oxidative damage surge at middle age. Second, IL33-deficiency impairs DSB repair de nova. Thus, IL33-ST2-NFκB axis is vital for up-regulation of DSB repair in neurons. Involvement of NFκB pathway in DSB repair in various tissues including aged brains has been reported [40-42]. Neuroinflammation has recently been considered as a cause or a promotor for Alzheimer’s disease or related dementia. However, it is unlikely that IL33 is involved in dementia through promoting neuroinflammation in our model.
Dysfunction Of Interleukin33-ST2-Nfkb Axis Impair Neuronal Rejuvenation
Our study has shown that IL33 deficiency impairs initiation of DSB repair. The next question is whether IL33-ST2-NFκB axis also controls other rejuvenation mechanisms in stressed neurons simultaneously? Beyond this possibility, another one is that accumulation of unrepaired DSB causes genomic instability that interrupts normal neuronal function as well all or some rejuvenation mechanisms. This question remains to be answer. How to answer this question? We believe that IL33 deficient mice is model to facilitate our effort to answer the question with the following reasons. First, IL33 deficient mice causes dysfunction in two other important rejuvenation mechanisms: autophagy and glymphatic drainage. Second, defects in those mechanisms have been linked to Alzheimer’s or related dementias.
Thus, IL33-deficient mice develop chronic neurodegenerative diseases, following a similar course to, and shares hallmarks with sporadic Alzheimer’s disease: massive synapse and neurite loss in the cortex and hippocampus after middle age, abnormal tau deposition in neurons and chronic neurodegeneration, and finally cognition impairment and memory loss at late life after a long asymptomatic period [17]. Third, although two types of Alzheimer’s diseases in humans display similar pathological characteristics (i.e. tau deposition and amyloid plaques), sporadic late on-set type is unrelated to genomic mutation. From this point of view, neurodegenerative disease in IL33-deificent mice mimics late onset type better than other mutation-based Alzheimer’s models. It needs to emphasize that late-onset type, but not early onset one, is among age-related dementias.
Thus, this model may be used to answer the question if IL33-ST2-NFκB axis also controls other rejuvenation mechanisms. One may argue that IL33 deficient mice do not develop amyloid plaques, one of the most important hallmarks of Alzheimer’s disease. In fact, unlike humans or other species, murine APP gene lacks structural base for formation of plaques [43]. However, abnormal tau deposition in IL33-deficienct brains may have indicated that a lack of neuronal rejuvenation has created an internal environment that promotes protein denaturation, accumulation and aggregation. We have shown that reduced glymphatic drainage in IL33 deficient mice accelerates abnormal tau accumulation in brains [44]. Our recent preliminary data showed accumulation of amyloid plaque in IL33 deficient mice transgened with human wild type APP.
Conclusive Remark
Recent studies including ours have given the following potential scenario for development of senile or age-related dementias by deficiency of IL33-NFκB axis in neurons (Fig. 2). In normal individuals, immediate repair of DSBs during oxidative surge ensures normal rejuvenation process for stressed neurons toward a full recovery. On the other hand, accumulation of unrepaired DSBs due to IL33-NFκB deficiency disrupts some or all subsequent rejuvenation mechanisms. Massive DSBs should otherwise have induced apoptosis in any other types of cells, but not in those neurons [17] (Figure 2).
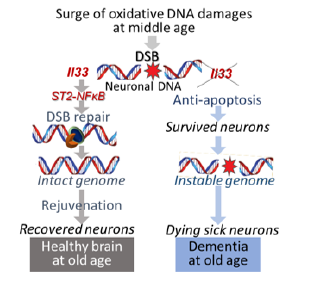
Figure 2: Lou Deficiency in Regulation of DNA Damage Repair in Neurons May Open Long Path at Middle Age to Dementias at Old Age.
It raises an interesting question why neurons with massive DSBs due to failed DSB repair mechanism do not go to apoptosis, or, how they can survive for a long time? In fact, it also is a question for human Alzheimer’s disease why do neurons avoid rapid apoptotic death despite of many DSBs? We have not had a full answer yet for it. Our recent study in IL33 deficiency model revealed a strong re-expression of apoptosome protein Apaf1, which has been silenced in mature neurons, after oxidative damage surge together with expression of several pro- and anti-apoptotic genes. It suggests a death and life struggle by stressed neurons. Complicated interactions among those proteins may avoid apoptosis in neurons but with a significant price, i.e. loss of their functionality by shrinking or withdrawing neurites and synapse as seen in early stage of Alzheimer’s in humans and middle aged IL33 deficient mice [17]. Since those neurons never recover, they eventually undergo chronic death or losing all functions.
Acknowledgements
This work was supported by NIH R21AG067311 (to YL), NIH R01DK077857 (to YL) and NIH R01HD049613 (to YL).
Conflict of Interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Anderson AJ, Su H, Cotman CW (1996) DNA damage and apoptosis in Alzheimer's disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci 16:1710-1719.
- Coppede F, Migliore L (2009) DNA damage and repair in Alzheimer's disease. Curr. Alzheimer Res 6: 36-47.
- Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19: 983-997.
- Frake RA, Ricketts T, Menzies FM, Rubinsztein DC (2015) Autophagy and neurodegeneration. J Clin Invest 125: 65-74.
- Suberbielle E, Djukic B, Evans M, Kim DH, Taneja P, et al. (2015) DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat Commun 6: 8897.
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, et al. (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA 101(7): 2070-2075.
- Barnett A, Brewer GJ (2011) Autophagy in aging and Alzheimer's disease: pathologic or protective? J Alzheimers Dis. 25: 385-394.
- Swerdlow RH (2011) Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta 1812(12): 1630-1639.
- Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16(6): 345-357.
- Wyss-Coray T (2016) Ageing, neurodegeneration and brain rejuvenation. Nature 539(7628):180-186.
- Schumacher B, Pothof J, Vijg J, et al. (2021) The central role of DNA damage in the ageing process. Nature 592: 695-703.
- Holmes GE, Bernstein C, Bernstein H (1992) Oxidative and other DNA damages as the basis of aging: a review. Mutat Res 275 (3-6): 305-315.
- Duke CG, Kennedy AJ, Gavin CF, Day JJ, Sweatt JD, et al. (2017) Experience dependent epigenomic reorganization in the hippocampus. Learning & Memory 24 (7): 278-288.
- Halder R, Hennion M, Vidal RO, Shomroni O, Rahman RU, Rajput A, et al. (2016) DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nature Neuroscience 19 (1): 102-110.
- Lu T, Pan Y, Kao SY, Li C, Kohane I, et al. (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429(6994): 883-891.
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, et al. (2001) Dose oxidative damage to DNA increase with age? Proc Natl Acad Sci USA 98(18): 10469-10474.
- Carlock C, Wu J, Shim J, Moreno-Gonzalez I, Pitcher M, et al. (2017) Interleukin33 deficiency causes tau abnormality and neurodegeneration with Alzheimer-like symptoms in aged mice. Trans Psychiatry 7(8): e1191.
- Lou Y (2021) Role of interleukin33 in rejuvenation of aged neurons and age-related dementias. Neurosci Insights16.
- Bender AR, Völkle MC, Raz N (2016) Differential aging of cerebral white matter in middle-aged and older adults: A seven-year follow-up. NeuroImage 125: 74-83.
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, et al. (2001) Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22(4): 581-594.
- Reid DA, Reed PJ, Schlachetzki JCM, Nitulescu II, Chou G, et al. (2021) Incorporation of a nucleoside analog maps genome repair sites in postmitotic human neurons. Science 372(6537): 91-94.
- Mark A. Lovell, William R. Markesbery (2007) Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res 35(22): 7497-7504.
- Lin X, Kapoor A, Gu Y, Chow MJ, Peng J, et al. (2020) Contributions of DNA Damage to Alzheimer's Disease. Int J Mol Sci 21(5): 1666.
- Shanbhag NM, Evans MD, Mao W, Nana AL, Seeley WW, et al. (2019) Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol Commun 7(1): 77.
- Pao PC, Patnaik D, Watson LA, Gao F, Pan L, et al. (2020) HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat Commun 11: 2484.
- Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, et al. (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163(1): 69-79.
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, et al. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23(5): 479-490.
- Yasuoka S, Kawanokuchi J, Parajuli B, Suzumura A (2011) Production and functions of IL-33 in the central nervous system. Brain Res 1385: 8-17.
- Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, et al. (2012) Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol 188: 3488-3495.
- Wicher G, Husic E, Nilsson G, Forsberg-Nilsson K (2013) Developmental expression of IL-33 in the mouse brain. Neurosci Lett 555: 171-176.
- Martin NT, Martin MU (2016) Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 17(2): 122-131.
- Li M, Li Y, Liu X, Gao X, Wang Y, et al. (2012) IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neuroimmunol 247: 25-31.
- Stojanovic B, Milovanovic J, Arsenijevic A, Milovanovic M, Arsenijevic N, et al. (2014) IL-33/ST2 axis mediates resistance to EAE by promoting regulatory B and tolerogenic dendritic cells. Neuroimmunol 275((1-2): 11-12.
- Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J, et al. (2015) The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS Injury. Neuron 85(4): 703-709.
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, et al. (2007) IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 117(6): 1538-1549.
- Chapuis J, Hot D, Hansmannel F, Kerdraon O, Ferreira S, et al. (2009) Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer's disease. Mol Psychiatry 14(11): 1004-1016.
- Fu AK, Hung KW, Yuen MY, Zhou XD, Mak S, et al. (2016) IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc Natl Acad Sci USA 113(19): E2705-2713.
- Wu J, Carlock C, Zhou C, Nakae S, Hicks J, et al. (2015) Interleukin33 is required for disposal of unnecessary cells during ovarian atresia through regulation of autophagy and macrophage migration. J Immunol 194(5): 2140-2147.
- Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, et al. (2014) Alzheimer's disease: evidence for the expression of interleukin-33 and its receptor ST2 in the brain. J Alzheimers Dis 40(2): 297-308.
- Volcic M, Karl S, Baumann B, Salles D, Daniel P, et al. (2012) NF-κB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic Acids Res 40(1): 181-95.
- Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, et al. (2012) NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 122(7): 2601-2612.
- Kraft D, Rall M, Volcic M, Metzler E, Groo A, et al. (2015) NF-κB-dependent DNA damage-signaling differentially regulates DNA double-strand break repair mechanisms in immature and mature human hematopoietic cells. Leukemia 29(7): 1543-1554.
- Gotz J, Ittner LM (2008) Animal models of Alzheimer’s disease and frontotemporal dementia. Rev Neurosci. 9(7): 532-544.
- Wu J, Shim J, Carlock C, Moreno-Gonzalez I, Barichello T, et al. (2021) Interleukin33 regulates aquaporin4 expression in astrocytes and glymphatic drainage of abnormal tau from brains. Mol Psychiatry 26(10): 5912-5924.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.